Best Describes the Properties of Covalent Compounds
Methane CH4 is a covalent compound with exactly 5 atoms that are linked by covalent bonds. Metallic bonds are malleable and ductile while covalent bonds and ionic bonds non-malleable and non-ductile.
Covalent Compounds Covalent Bond Properties Examples With Videos
A feature of organic compounds not found in inorganic compounds is the presence of.

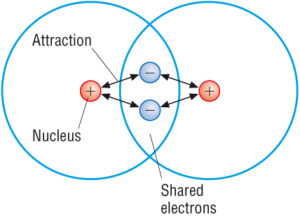
. Ionic compounds have higher melting points than covalent compounds. A chemical bond that involves sharing a pair of electrons between atoms in a molecule. When placed at room temperature the substance quickly turned into a liquid.
Carbon has four valence electrons so it can achieve a full outer energy level by forming four covalent bonds. An _____ is the smallest particle of a covalent compound that still has the properties of the compound. Covalent compounds tend to be more flammable than ionic compounds Covalent compounds dont conduct electricity in water.
Properties of Covalent Compounds. Click card to see definition. They are all hard and do not conduct electricity because there are no free charges that can move.
Melting and boiling points of the covalent bond are low unlike the metallic bonds and ionic bonds which have higher. C soluble hard high melting point and electrical conductivity. When it bonds only with hydrogen it forms compounds called hydrocarbons.
Covalent compounds are soft and squishy compared to ionic compounds anyway. How are compounds with metallic bonds similar to ionic compounds. Tap card to see definition.
This compound is most likely which of the following. Examples include diamond quartz SiO. Which is the rarest property among compounds.
Here is a short list of the main properties of covalent compounds. As compared to a covalent compound a soluble soft low melting point and electrical conductivity. A covalent compound because it has a low melting.
A solid compound in a sealed container was kept at a very low temperature in a freezer. Some properties of ionic compounds are high melting points solid in room temperature and they are brittle. What are the properties of a covalent network.
Carbon can form single double or triple covalent bonds with other carbon atomsNov 14 2012. While the ions in an ionic compound are strongly attracted to each other covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them. Covalent compounds bonds are not broken apart when placed in water.
While the ions in an ionic compound are strongly attracted to each other covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them. The electrons in covalent compounds are less mobile than the electrons in ionic compounds. Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds.
Most covalent compounds have relatively low melting points and boiling points. They do not dissolve. Most covalent compounds have relatively low melting points and boiling points.
Low melting points and boiling points. Most covalent compounds have relatively low melting points and boiling points. We draw this covalent bonding as a Lewis structure see diagram.
Which statement best describes how the cash flow statement differs from the income statement. Low enthalpies of fusion and vaporization These properties are usually one or two orders of magnitude smaller than they are for ionic compounds. Covalent compounds arent usually very soluble in water.
Covalent compounds tend to be more flammable than ionic compounds. Covalent compounds generally have much lower melting and boiling points than ionic compounds. Is Diamond ionic or covalent.
B insoluble hard low melting point and non-conductive. D insoluble soft low melting point and non-conductive. All covalent network structures have very high melting points and boiling points because many strong covalent bonds need to be broken.
The methane molecule is this group of 5 atoms connected as such. Properties of Covalent Molecular Compounds. Ionic compounds are composed of a metal and a nonmetal while covalent compounds are composed of all nonmetals.
Properties Of Covalent Compounds Homework Properties of Covalent Compounds. Water has many exceptional and useful properties. Which of the following list of properties best describes an ionic compound.
There are four bonds from a central carbon C linking or bonding it to four hydrogen atoms H. What statement best describes the properties of ionic compounds. Is CH4 a covalent compound.
A solid that is extremely hard that has a very high melting point and that will not conduct electricity either as a solid or when molten is held together by a continuous three-dimensional network of covalent bonds. Which statement best describes a covalent bond.

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Which Of The Following Phrases Best Describes Process Focus In 2022 Process The Selection Focus

Covalent Compounds Covalent Bond Properties Examples With Videos
Comments
Post a Comment